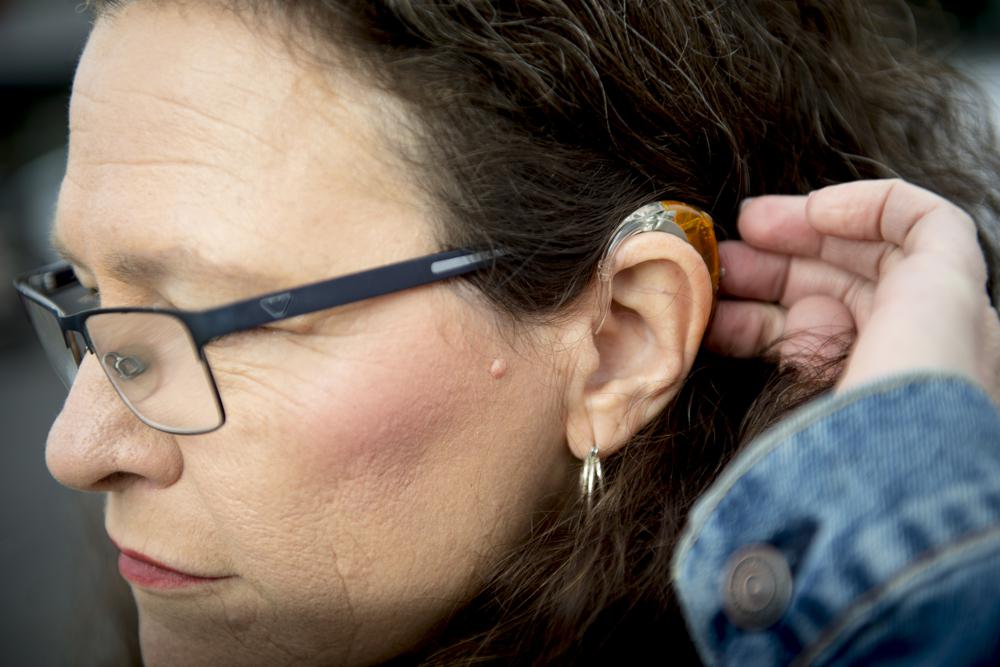
Millions of Americans will be able to buy hearing aids without a prescription later this fall, under a long-awaited rule finalized Tuesday.
The Food and Drug Administration said the new regulation cuts red tape by creating a new class of hearing aids that don’t require a medical exam, a prescription and other specialty evaluations. Instead the devices will be sold online or over-the-counter at pharmacies and other retail stores.
The devices are intended for adults with mild to moderate hearing problems. The FDA estimates that nearly 30 million adults could potentially benefit from hearing aid use, but only about one-fifth of people with hearing problems use the devices currently. The FDA first proposed the rule last October. The new rule will take effect in mid-October.
Biden administration officials highlighted the potential cost savings.
“Today’s action by the FDA represents a significant milestone in making hearing aids more cost-effective and accessible,” Health and Human Services Secretary Xavier Becerra, said in a statement.
The move follows years of pressure from medical experts and consumer advocates to make the devices cheaper and easier to get.
Cost is a big obstacle now. Between the device itself and fitting services, Americans can pay more than $5,000 to get a hearing aid. Insurance coverage is very limited, and Medicare doesn’t pay for hearing aids, only diagnostic tests.
The new over-the-counter status won’t apply to devices for more severe hearing loss, which will remain prescription only.
Consumer electronic companies for years have produced lower-cost “personal sound amplification” devices, but U.S. regulations bar them from being marketed as hearing aids and they do not undergo FDA review. The new rule makes explicit that those devices are not alternatives to FDA-vetted hearing aids. Companies that market them inappropriately could face penalties, such as fines or product seizures.
The FDA said it changed several parts of its initial proposal in response to public comments, including clarifying how the federal rule will impact state regulations on hearing aids.
Once the federal rule takes effect, traditional manufacturers are expected to begin selling cheaper, direct-to-consumer models. Eventually, advocates predict the hearing aid market will resemble eye care, where consumers can choose between drugstore reading glasses or prescription bifocals.
Tuesday’s announcement follows prodding from medical committees and Congress, which in 2017 instructed the agency to lay out a plan for over-the-counter hearing devices.
(AP)










One Response
Does that mean they will no longer be covered by health insurance, as they are for many if not most people with private health insurance. Health insurance plans rarely cover anything you can buy on your own without a formal prescription for a recognized health care provider.
That also raises issues since hearing loss is a condition that can indicate something serious,so deciding that hearing loss isn’t something treated by medical professional might be counter-productive.