A statement from Florida Governor Ron DeSantis has many worried that lifesaving monoclonal antibody treatments will no longer be available across the United States. However, this is not the case.
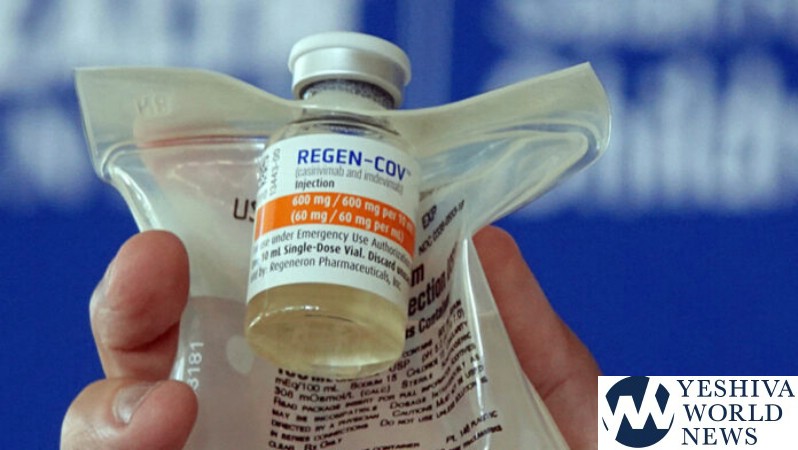
The FDA on Monday pulled its emergency use authorization for the antibody treatments from two companies – Regeneron and Eli Lilly – because those treatments have been shown to be far less effective against Omicron than they were with previous strains, such as Delta.
This prompted Florida Governor DeSantis to condemn the Biden administration, saying that the FDA didn’t have “a shred of clinical data to support this action” and that “Biden has forced trained medical professionals to choose between treating their patients or breaking the law.”
However, it is important to understand that there is a third monoclonal antibody treatment – developed and produced by GlaxoSmithKline – that is still authorized for use and has shown to be highly effective against Omicron infections.
YWN spoke with the Covid Plasma Initiative, who confirmed that they are still able to send patients for monoclonal antibody treatments, and that there is supply of the treatments available.
If you have become infected with Covid-19, it is important that you research your treatment options to ensure optimal outcomes. Yes, monoclonal antibody treatments are still available, and the Covid Plasma Initiative is still helping people get them.
If you become infected with Covid-19, call the Covid Plasma Initiative at 828-475-2762
(YWN World Headquarters – NYC)











10 Responses
When YWN has to explain to its worried audience that DeSantis is a terrible source of info on covid.
Beyond stupid for DeSantis. He discourages using the Trump Warp Speed covid vaccines, which so far have shown varying degrees of efficacy on reducing infection and degree of illness but now wants to retain access to two drugs that have shown NOT to be effective and is silent about the one monoclonal antibody treatment that has been shown effective and is available. He should get his head out from his behind and realize there may be some Floridians who are stupid enough to follow his meshugah guidance.
As far as I know, Covid Plasma Initiative does not operate in Florida, and therefore has no first-hand information on what is happening there. If DeSantis says they had to close the centers because there isn’t enough of the one remaining drug available, his word should be good enough, especially when pitted against this administration which lies routinely.
It seems to be more political posturing.
And wait it might kill you since it’s only an emergency authorization. (For all you antivaxxers)
DeSantis said that the FDA dropped Regeneron “without a shred of clinical evidence”, and that is clearly the truth (you can be sure if there were any such evidence, even barely-qualified evidence, it would be trumpeted from the rooftops). DeSantis’ concern is that this action by the corrupt FDA, inspired no doubt by some under-the-table feud between Regeneron and the FDA (remember that this is the drug that saved Donald Trump personally in 2020, and that one of the company’s founders is a long-time good friend of Trump), is limiting the availability of the life-saving drug, and that is clearly also true (a bunch of monoclonal therapy distribution centers had to close in Florida because of this, as the article states). So the “Explainer” explains nothing, unless you’re still drinking the cool aid…
Contorted conspiracy nonsense spouted by frumwhere continues. And if the FDA approved it, you would be saying it’s experimental and could kill you and big pharma is telling the fda to approve it so they can charge $1000 a dose.
What DeSantis is saying: lack of _clinical_ data. CDC rescinded authorization based on _lab_ tests that showed Regeneron ineffective against Omicron. It seems likely that the drug that does not help in the lab will not help to the patients. Regeneron is currently testing an Omicron version, so it will be back in play in 1-2 months.
Delta is still 10% of the world Covid, so it makes sense to send Regeneron to places where it works rather than trying to find a way to make it barely work with omicron.
Frumwhere: Giving you the benefit of the doubt, READ the FDA rationale for removing the EUAs for these two MA drugs in favor of the GSK MA therapy which HAS been shown to have much greater efficacy. The studies cited by the FDA are peer reviewed and reasonably definitive as to the failure of the two MA drugs to show statistically significant benefits, notwithstanding some anecdotal reports about Trump and his chevrah being “saved”.
However, thank you for your conspiracy theories about anti-trump sentiment driving the FDA decision which are highly entertaining on an otherwise slow news day.
FRUMWHERE YOU ARE TOTALLY RIGHT…
THE REST OF YOU…. CONTINUE DRINKING THE KOOL-AID.
Gadol > reasonably definitive as to the failure of the two MA drugs to show statistically significant benefits, notwithstanding some anecdotal reports about Trump and his chevrah being “saved”.
to clarify – failure is for Omicron. They are effective for Delta, but most of covid in US is Omicron and they are not doing testing on every patient. Still, there are several remote states where Delta is still high, and so are other countries where the drug can go and not be wasted. Omicron version seem to be on the way in next 2 months.