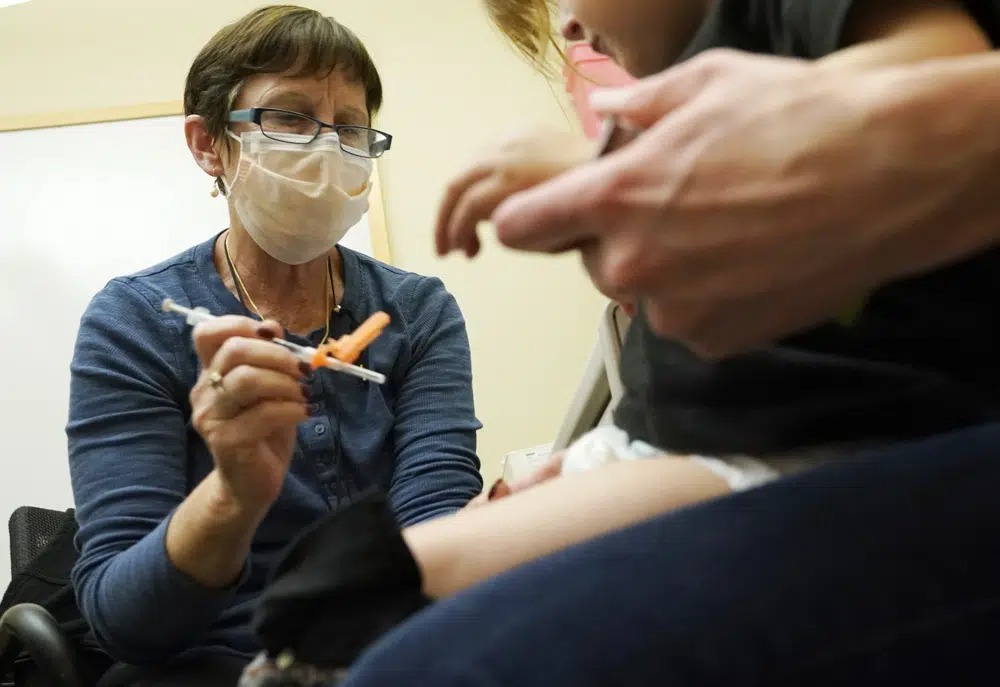
U.S. regulators on Thursday cleared doses of the updated COVID-19 vaccines for children younger than age 5.
The Food and Drug Administration’s decision aims to better protect the littlest kids from severe COVID-19 at a time when children’s hospitals already are packed with tots suffering from a variety of respiratory illnesses.
Omicron-targeted booster shots made by Moderna and rival Pfizer already were open to everyone 5 and older.
The FDA now has cleared their use in tots starting at age 6 months — but just who is eligible depends on what vaccinations they’ve already had, and which kind. Few youngsters have gotten the full primary series since shots for the littlest kids began in June.
The FDA decided that:
–Children under age 6 who’ve already gotten two original doses of Moderna’s COVID-19 vaccine can get a single booster of Moderna’s updated formula if it’s been at least two months since their last shot.
–Pfizer’s vaccine requires three initial doses for tots under age 5 — and those who haven’t finished that vaccination series will get the original formula for the first two shots and the omicron-targeted version for their third shot.
–Children under 5 who already got all three Pfizer doses aren’t yet eligible for an updated booster. Data expected next month should help the FDA determine if and when those tots need the omicron-targeted booster.
The Centers for Disease Control and Prevention is expected to sign off soon, the final step for shots to begin.
Just 3% of tots under 2 and nearly 5% of those 2 to 4 have gotten their primary doses so far, according to the CDC.
“Vaccines remain the best defense against the most devastating consequences of disease caused by the currently circulating omicron variant,” FDA vaccine chief Dr. Peter Marks said in a statement.
The updated vaccines from Moderna and Pfizer are combination shots, containing half the original vaccine and half tweaked to match the BA.4 and BA.5 omicron strains that until recently were dominant. Now BA.5 descendants are responsible for most COVID-19 cases.
The CDC last month released the first real-world data showing that an updated booster, using either company’s version, does offer added protection to adults. The analysis found the greatest benefit was in people who’d never had a prior booster, just two doses of the original COVID-19 vaccine — but that even those who’d had a summertime dose were more protected than if they’d skipped the newest shot.
(AP)










One Response
If you needed another wake up call that the FDA works for industry and not for your kids, read the above article. This new updated booster, for the record, has never been tested in humans. The safety trials included 8 mice. Furthermore, in order to be eligible for this latest and greatest, one must first receive TWO of the primary vaccines, for a strain that is no longer in circulation. And then they can have the honor of receiving the newest latest and updated booster, also for a strain which is dying quickly out of circulation. The problem with vaccines for viruses like colds and flu is that as quickly as they can develop as new vaccine strain, it can’t keep up with the speed that the virus mutates out in the real world. Add to the mess the fact that the vaccine doesn’t even protect against transmission, and has many scary side effects to boot. Why ks this even still on the market???